[Introduction] Allogeneic peripheral blood stem cell transplantation (allo-PBSCT) is a curative treatment for hematological malignancies but has a risk of a chronic graft-versus-host disease (GVHD) compared to other donor sources. Post-transplant cyclophosphamide (PTCY) has been spread rapidly worldwide from HLA haploidentical-SCT (Haplo-SCT) to HLA matched-SCT. Although PTCY was theoretically thought to preserve non-alloreactive T cells which can contribute to fight infection, recent studies showed that PTCY was associated with the increased incidence of cytomegalovirus (CMV) infection. Letermovir (LTV), a novel anti-CMV agent, was useful for the prevention of CMV reactivation after SCT including Haplo-SCT using PTCY. On the other hand, low-dose antithymocyte globulin (ATG) is an alternative and useful option for GVHD prophylaxis in allo-PBSCT and often used in Japan. However, the risk of post-transplant CMV reactivation and the protective effect of LTV when using low-dose ATG remain to be elucidated.
[Methods] We retrospectively analyzed 222 recipients who received allo-PBSCT using PTCY or low-dose ATG at Hokkaido University Hospital from January 2013 to July 2021. Of the 121 patients who underwent PBSCT with PTCY, 69 (all patients underwent Haplo-PBSCT) were without LTV prophylaxis and 52 (34 patients, Haplo-PBSCT) were with LTV. On the other hand, of the 101 patients who underwent PBSCT with low-dose ATG, 66 (12 patients, Haplo-PBSCT) were without LTV and 35 (2 patients, Haplo-PBSCT) were with LTV. LTV was administered on the day 0 at a dosage of 480 mg daily. All patients were monitored for CMV reactivation by using the anti-CMV pp65 monoclonal antibody HRP-C7 assay at least weekly from neutrophil engraftment to discharge and after discharge monthly until day 180 after SCT. CMV reactivation was defined as the start of CMV preemptive therapy, generally initiated when there are 2 or more CMV antigen positive cells per 50000 white blood cells. Moreover, CMV disease was defined by organ dysfunction attributable to CMV.
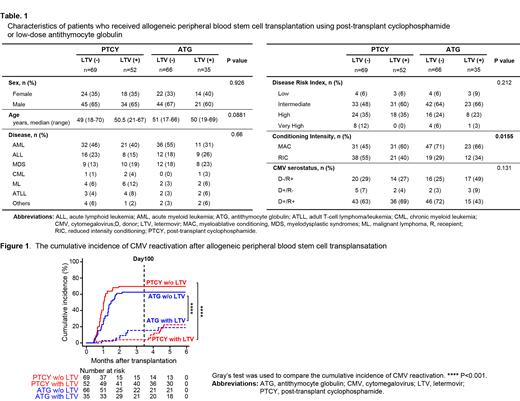
[Results] Baseline characteristics of the patients are summarized in Table 1. Although more patients in ATG group compared to PTCY group received myeloablative conditioning regimen significantly, there was no significant difference between the groups in terms of sex, age, underlying disease, disease risk at SCT, and CMV serostatus. GVHD prophylaxis in PTCY consisted of CY (40-50 mg/kg on day 3 and 4), tacrolimus (from day 5), and mycophenolate mofetil (from day 5), whereas that in ATG consisted of ATG at a total dose of 2-3 mg/kg around day -2, tacrolimus (from day -1) and short-term methotrexate. LTV prophylaxis significantly reduced the cumulative incidence of CMV reactivation at 180 days after SCT in PTCY group (69.6% without LTV vs. 22.4% with LTV, p<0.001) and ATG group (62.6% without LTV vs. 18.9% with LTV, p<0.001; Figure 1.). Importantly, although CMV disease were occurred in 2 patients in PTCY (1 gastritis and 1 retinitis) and 8 focuses in 7 patients (3 pneumoniae, 3 enteritis, 1 retinitis, and 1 glossitis) in ATG without LTV prophylaxis, none of the patients receiving LTV developed CMV disease in both group. Moreover, LTV did not affect overall survival, the cumulative incidence of non-relapse mortality, relapse, acute GVHD, and engraftment of neutrophil and platelet. Interestingly, the cases of early CMV reactivation within 100 days after SCT under LTV prophylaxis was significantly more frequent in ATG than in PTCY (p=0.0345).
[Conclusion] CMV reactivation occurred with similar frequency after allo-PBSCT with low-dose ATG as after that with PTCY. LTV was effective for prevention of CMV reactivation in both GVHD prophylaxis methods. CMV breakthrough reactivation may be likely to occur in PBSCT with low-dose ATG compared to that with PTCY even under LTV prophylaxis, and careful CMV monitoring may be required.
Disclosures
Goto:Novartis: Honoraria; Chugai: Honoraria; Kyowa Kirin: Honoraria, Research Funding; Bristol-Myers Squibb: Research Funding; SymBio: Research Funding. Nakagawa:Takeda Pharmaceutical Company Limited: Honoraria, Research Funding; AbbVie Inc.: Research Funding; Meiji Seika pharma: Honoraria; Astrazeneca: Honoraria; Mundipharma: Honoraria. Hashimoto:Astellas Pharma: Honoraria; Daiichi Sankyo Inc: Honoraria; Ono Pharma: Honoraria; Janssen Pharma: Honoraria; Kyowa-Kirin: Honoraria; Chugai Pharmaceutical: Honoraria; LUCA Science: Patents & Royalties. Teshima:Chugai: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; Fuji Pharma: Research Funding; NIPPON SHINYAKU: Honoraria, Research Funding; Asahi Kasei Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai: Research Funding; Sumitomo Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ONO: Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; SHIONOGI: Research Funding; Priothera SAS: Research Funding; LUCA Science: Research Funding; Otsuka: Research Funding; AbbVie: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria; Merck Sharp & Dohme: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Meiji Seika Pharma: Membership on an entity's Board of Directors or advisory committees; DAIICHI SANKYO: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Roche Diagnostics: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal